
Clinical Adjudication
Improve accuracy and consistency of clinical endpoint
adjudication data and streamline CEC review

Improve accuracy and consistency of clinical endpoint
adjudication data and streamline CEC review
Prevail’s advanced CEC tools support the centralized decision-making process for safety and efficacy endpoints to standardize outcomes and optimize data quality, driving study success.
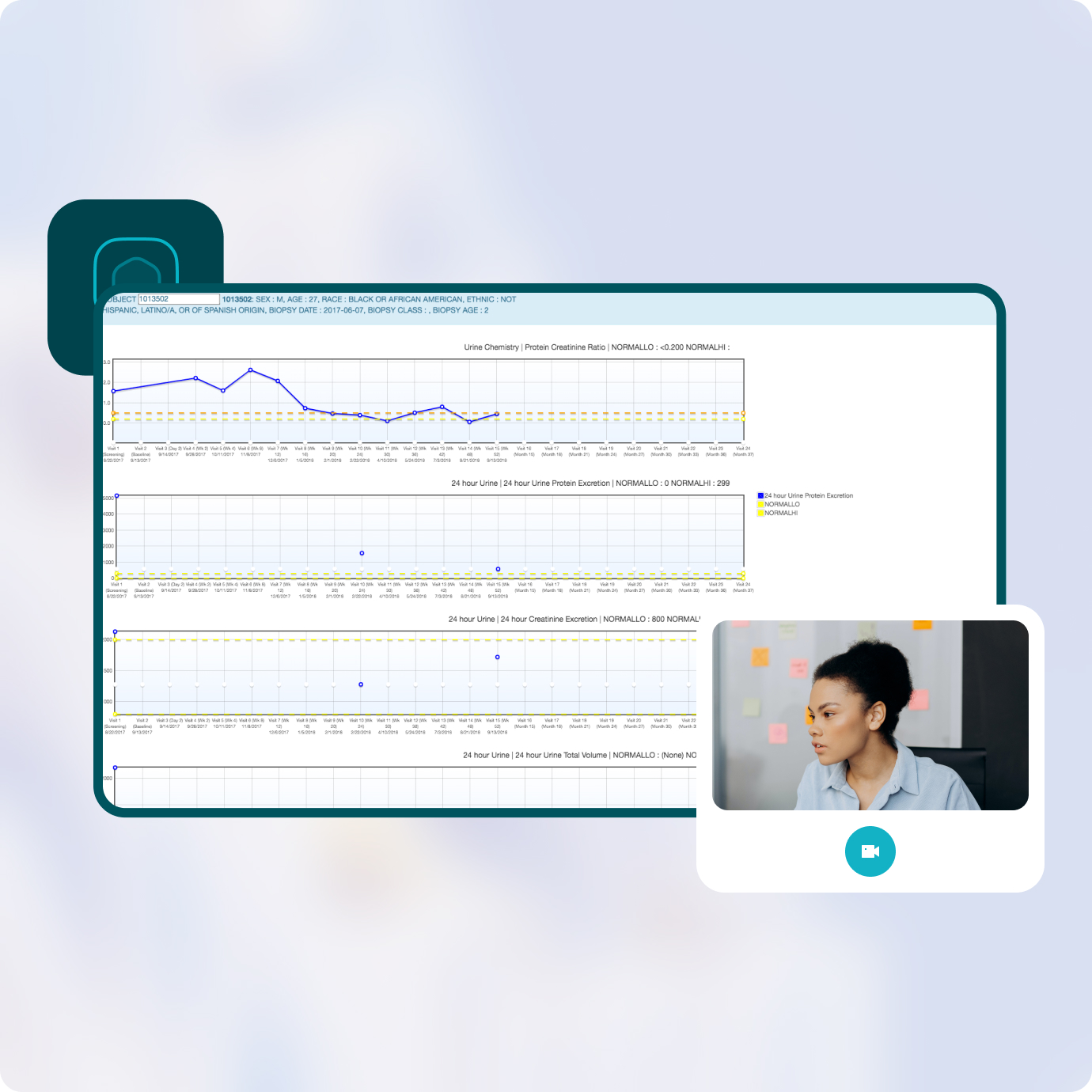
Prevail’s unique Visual Patient Profiles provide a comprehensive view containing only the data and time points required for CEC review, for single-study as well as cross-study review and analysis. Reviewers can apply their expertise to critical study data instead of struggling with software.
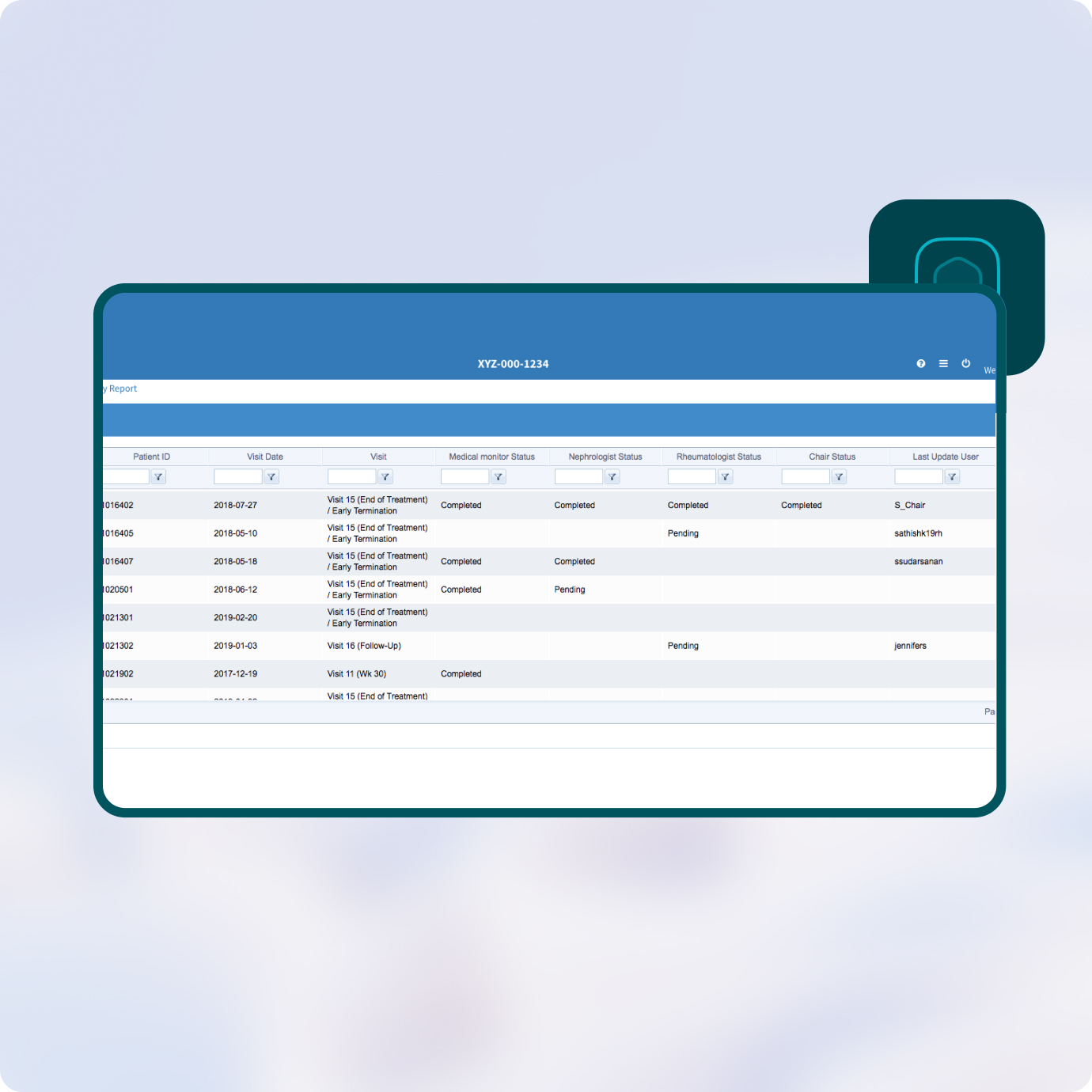
Prevail’s Clinical Endpoint Adjudication module has the flexibility to match your preferred workflow, whether it relates to alert messaging of evaluable patients, review cycles, data status, data presentation, or committee reporting.
Reports status of review by CEC members and their observations and findings.
Obtain valuable information on the results of the CEC review - including patient ID, reviewer role, detailed measures, findings and much more. Filter and export the summary report for use external to the system.
Patient folders link CEC members to tailored Visual Patient Profiles, with web-based forms for entry of adjudication information. Reviewers can easily access and complete review forms by simply clicking on the “CEC” tab within the Visual Patient Profile.
Learn how Prevail’s Clinical Adjudication can help make your study a success.

Ready to see how Prevail’s intelligent tools and workflows can help streamline clinical adjudication and CEC review?